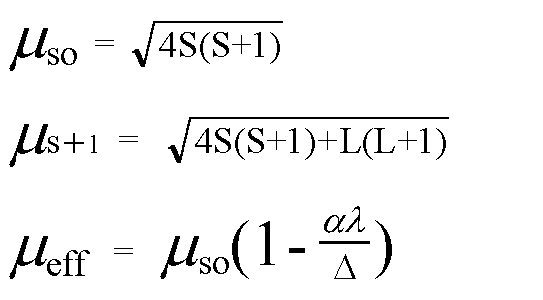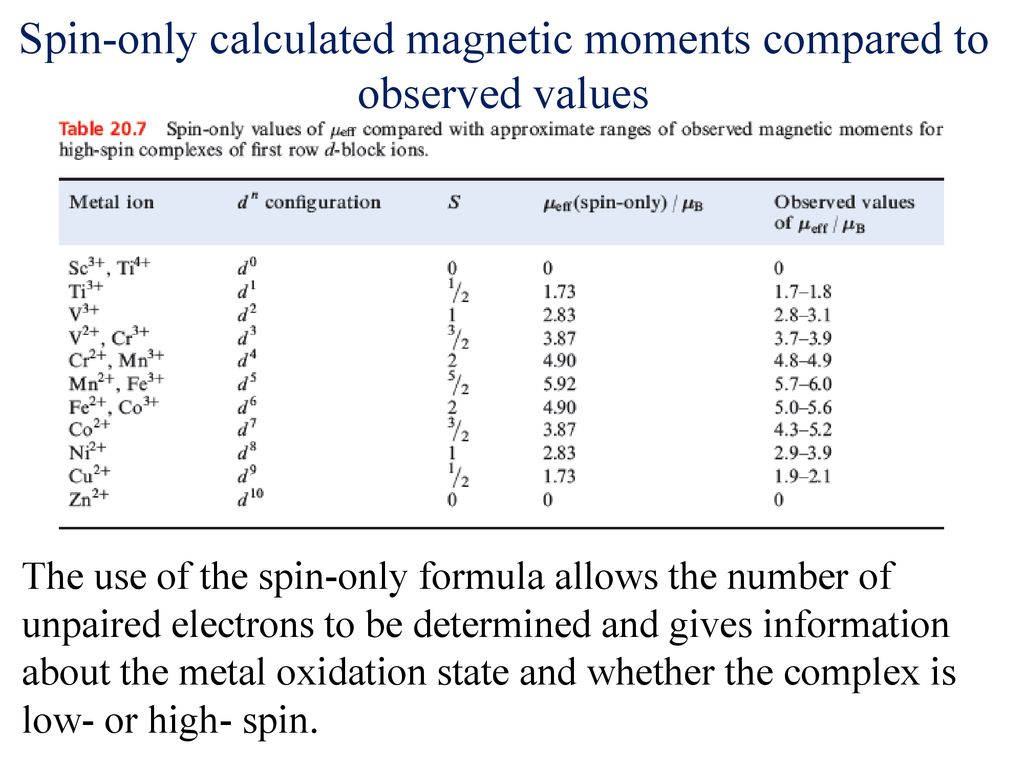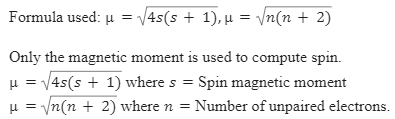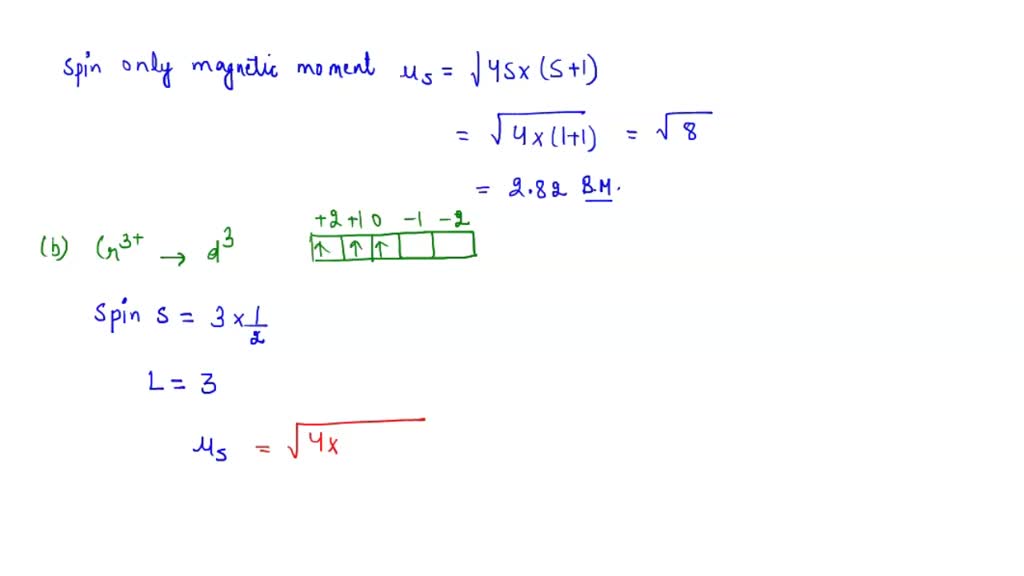
SOLVED: Calculate the magnetic moment of V 3+, Cr3+, Pr3+, Nd3+ according to the following instructions. (a) Consider spin-only magnetic moment in your calculation (b) Consider both spin and orbital moment in
The value of the 'spin only' magnetic moment for one of the following configurations is 2.84BM. The correct one is - Sarthaks eConnect | Largest Online Education Community
![The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/1263733.jpg)
The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-) (in units of BM ) respectively are
![The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/618499.jpg)
The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are
Calculate the spin only magnetic moment of La^3+. - Sarthaks eConnect | Largest Online Education Community
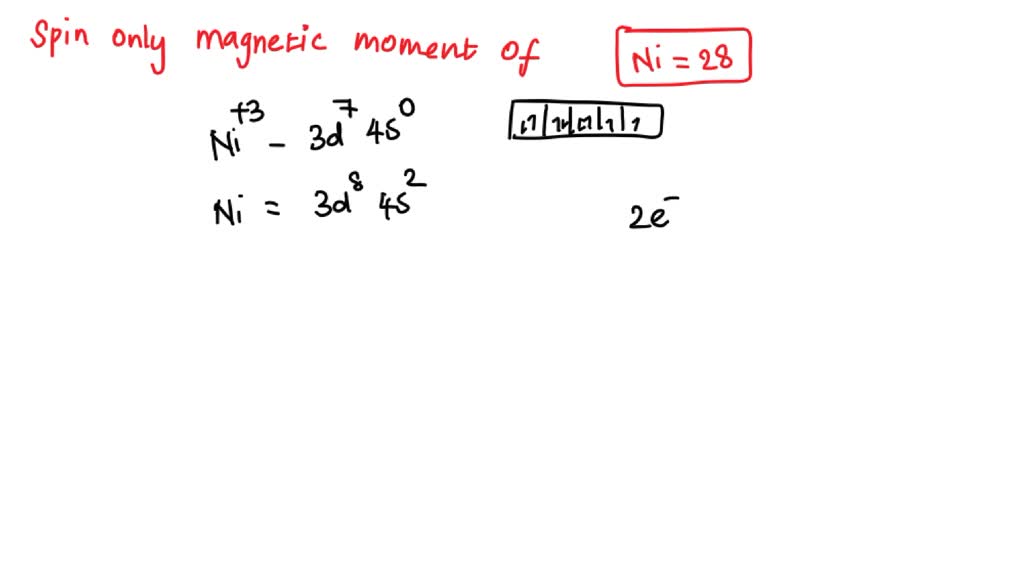
SOLVED: What is value of spin only magnetic moment of Ni (Z=28) in +3 oxidation state ? a)2.8 BM b) 3.1 BM c)0.0 BM d) 1.7 BM
![The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube](https://i.ytimg.com/vi/no_ZGBD689M/maxresdefault.jpg)
The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` - YouTube

The spin-only magnetic moment [in units of Bohr magneton, (mu(B) of Ni^(2+) in aqueous solution would be (atomic number of Ni=28)
![The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube](https://i.ytimg.com/vi/KVj56QvOV1I/maxresdefault.jpg)
The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube